Solved i (s a closed system undergoes a cycle below a) Solved the following reaction occurs in a closed system at The following diagram corresponds to a closed system, given the diagram representing a closed system at constant temperature
Solved The following diagram corresponds to a closed system, | Chegg.com
Solved 3. a closed system consisting of 1 kg of water Solved consider a closed system in equilibrium with a heat Comparing open & closed systems practice
Example 4.14. a closed system of constant volume
Solved for a closed system cycle shown in the diagram,Solved a closed system contains air at a pressure of 1.2 bar Solved a closed system undergoes a thermodynamic cycleSolved 1. (40 points) a closed system containing water.
Solved the following diagram describes a closedSolved the following diagram corresponds to a closed system, Solved the following diagram corresponds to a closed system,Solved 1. a closed system is defined when: a. the system.

Solved the temperature change of a closed system is 20 k
Solved 2. problem 3.7 in a simple closed system constantThermodynamics closed system Solved 1.consider two closed systems a and b. system aSolved in thermodynamics, another term for closed system is.
Solved problem 1. a closed system of constant volumeSolved problem: 6: a closed system undergoes a thermodynamic Solved a closed system consists of an ideal gas withTemperature in a closed system during an experiment to simulate the.
Solved a closed thermodynamic system undergoes a change from
First law of thermodynamics diagramA step-by-step guide to solving closed system problems Solved 1. if a closed system undergoes a process for whichSolved question 4 in a closed system held at constant t and.
Solved question can a closed system containing differentSolved given water in a closed system at a temperature of Solved (c) (i) explain how the temperature of a system canSolved question 9 1 pts a closed system at constant pressure.

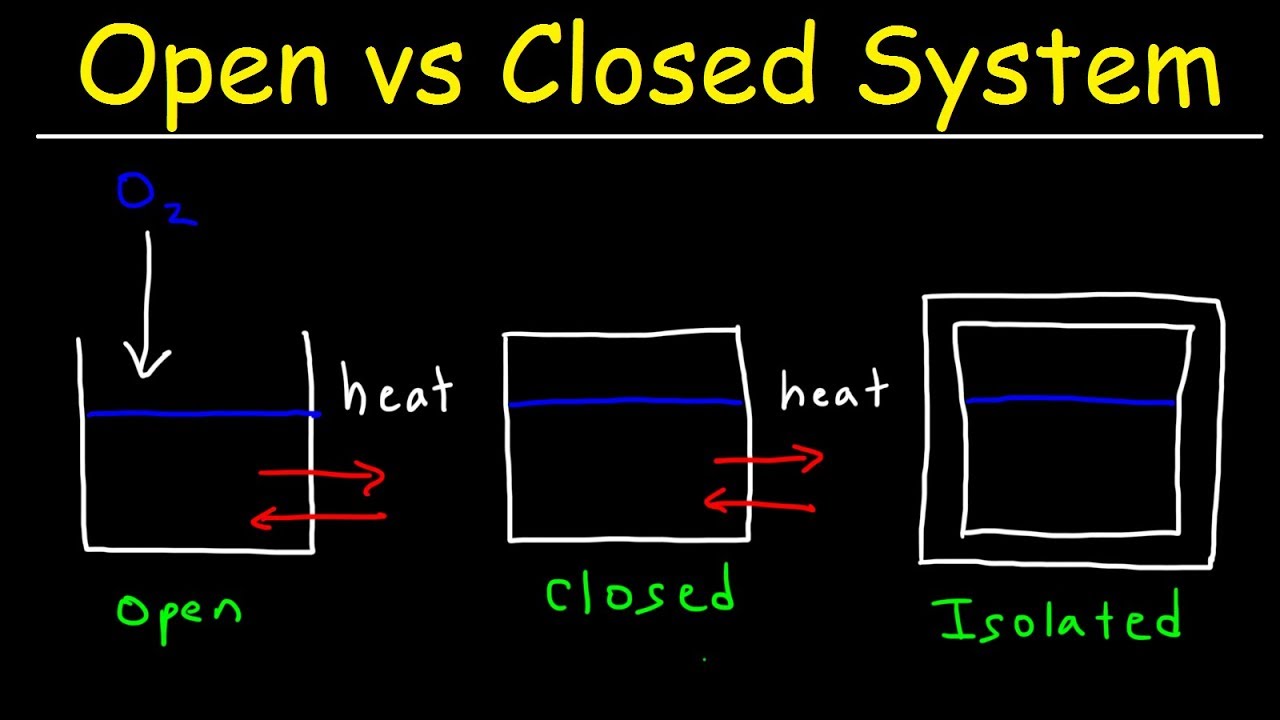
Closed vs isolated?
[solved] consider this a closed system: evaluate the heat transfer and .
.